技术专题
The Lancet:新抗体药物可完全清除银屑病症状
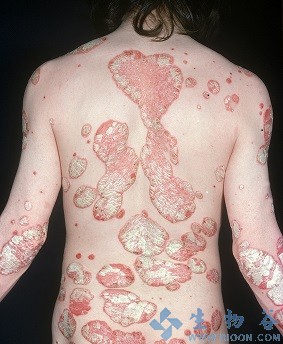
英国曼彻斯特的大学的研究人员进行了一项新的银屑病(又称牛皮癣)药物的临床试验。治疗12周后,40%的人的银屑病斑块的完全清除,超过90%的患者有显著改善。这篇研究在线发表在最近的柳叶刀杂志。
Ixekizumab是一种单克隆抗体,能够中和一种被称为白细胞介素-17A(IL-17A)的蛋白。这种蛋白传送信号到细胞中,诱发皮肤炎性反应。目前,这种蛋白质日益成为公认的银屑病中引起皮肤红点的原因。
该研究测试了2500位患有银屑病的患者。其中一半被给予了新药物——ixekizumab, 每隔两周或四周使用一次。另一半患者被给予了安慰剂或一种广泛使用的治疗银屑病的药物——依那西普。ixekizumab使用的实验组个体表现出快速和广泛的改善病情改善,效果远远超过安慰剂或依那西普。其中大约一半的患者在使用最快四周后表现出病情改善。十二周治疗后,高达71%的表现出较高水平的改善(使用银屑病面积和严重程度指数测量表)。
Chris Griffiths是曼切斯特大学医学和人文科学学院的教授,他领导了这项研究。他表示道:“银屑病对患者对生活的信心和自尊是一个重大打击。我们在这个实验中看到的不仅是疾病的物理方面清除,接受新药物的人们还报告了他们生活质量的显着改善,就因为他们感到的瘙痒减少,而且更加自信。”
根据Griffiths教授的说法,这个新药物正在改变银屑病患者的治疗前景。“治疗银屑病的目的是减少可见的症状,”他说,“新的药物正在迅速向我们展示了所有患者一个可以实现的目标。”
PMC
PMID
Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials.
Christopher E M Griffiths, MD et al
Summary
Background
Ixekizumab is a humanised monoclonal antibody against the proinflammatory cytokine interleukin 17A. We report two studies of ixekizumab compared with placebo or etanercept to assess the safety and efficacy of specifically targeting interleukin 17A in patients with widespread moderate-to-severe psoriasis.
Methods
In two prospective, double-blind, multicentre, phase 3 studies (UNCOVER-2 and UNCOVER-3), eligible patients were aged 18 years or older, had a confirmed diagnosis of chronic plaque psoriasis at least 6 months before baseline (randomisation), 10% or greater body-surface area involvement at both screening and baseline visits, at least a moderate clinical severity as measured by a static physician global assessment (sPGA) score of 3 or more, and a psoriasis area and severity index (PASI) score of 12. Participants were randomly assigned (1:2:2:2) by computer-generated random sequence with an interactive voice response system to receive subcutaneous placebo, etanercept (50 mg twice weekly), or one injection of 80 mg ixekizumab every 2 weeks, or every 4 weeks after a 160 mg starting dose. Blinding was maintained with a double-dummy design. Coprimary efficacy endpoints were proportions of patients achieving sPGA score 0 or 1 and 75% or greater improvement in PASI at week 12. Analysis was by intention to treat. These trials are registered with ClinicalTrials.gov, numbers NCT01597245 and NCT01646177.
Findings
Between May 30, 2012, and Dec 30, 2013, 1224 patients in UNCOVER-2 were randomly assigned to receive subcutaneous placebo (n=168), etanercept (n=358), or ixekizumab every 2 weeks (n=351) or every 4 weeks (n=347); between Aug 11, 2012, and Feb 27, 2014, 1346 patients in UNCOVER-3 were randomly assigned to receive placebo (n=193), etanercept (n=382), ixekizumab every 2 weeks (n=385), or ixekizumab every 4 weeks (n=386). At week 12, both primary endpoints were met in both studies. For UNCOVER-2 and UNCOVER-3 respectively, in the ixekizumab every 2 weeks group, PASI 75 was achieved by 315 (response rate 89·7%; [effect size 87·4% (97·5% CI 82·9–91·8) vs placebo; 48·1% (41·2–55·0) vs etanercept]) and 336 (87·3%; [80·0% (74·4–85·7) vs placebo; 33·9% (27·0–40·7) vs etanercept]) patients; in the ixekizumab every 4 weeks group, by 269 (77·5%; [75·1% (69·5–80·8) vs placebo; 35·9% (28·2–43·6) vs etanercept]) and 325 (84·2%; [76·9% (71·0–82·8) vs placebo; 30·8% (23·7–37·9) vs etanercept]) patients; in the placebo group, by four (2·4%) and 14 (7·3%) patients; and in the etanercept group by 149 (41·6%) and 204 (53·4%) patients (all p<0·0001 vs placebo or etanercept). In the ixekizumab every 2 weeks group, sPGA 0/1 was achieved by 292 (response rate 83·2%; [effect size 80·8% (97·5% CI 75·6–86·0) vs placebo; 47·2% (39·9–54·4) vs etanercept]) and 310 (80·5%; [73·8% (67·7–79·9) vs placebo; 38·9% (31·7–46·1) vs etanercept]) patients; in the ixekizumab every 4 weeks group by 253 (72·9%; [70·5% (64·6–76·5) vs placebo; 36·9% (29·1–44·7) vs etanercept]) and 291 (75·4%; [68·7% (62·3–75·0) vs placebo; 33·8% (26·3–41·3) vs etanercept]) patients; in the placebo group by four (2·4%) and 13 (6·7%) patients; and in the etanercept group by 129 (36·0%) and 159 (41·6%) patients (all p<0·0001 vs placebo or etanercept). In combined studies, serious adverse events were reported in 14 (1·9%) of 734 patients given ixekizumab every 2 weeks, 14 (1·9%) of 729 given ixekizumab every 4 weeks, seven (1·9%) of 360 given placebo, and 14 (1·9%) of 739 given etanercept; no deaths were noted.
Interpretation
Both ixekizumab dose regimens had greater efficacy than placebo and etanercept over 12 weeks in two independent studies. These studies show that selectively neutralising interleukin 17A with a high affinity antibody potentially gives patients with psoriasis a new and effective biological therapy option.
